It is denoted by the symbol �H� (the H in H2O is hydrogen). Hydrogen has one electron and one proton, its atomic number is 1. It is the first element in the periodic table. Hydrogen: Atomic number 1. No number means one atom. For CO2 there is one atom of carbon and two atomsof oxygen. For H2O, there is one atom of oxygen and two atoms of hydrogen. A molecule can be made of only one type of atom. Atom Molecule; Definition: An atom is the smallest and the most fundamental unit of any ordinary matter. Molecules comprise of two or even more atoms that are bound together by a chemical bond. Structure: An atom is the smallest of all types of particles possessing the same properties as the element. Molecules are a combination of a minimum of.
A Sweet Briar College Learning Resource |
Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. It has a role as an amphiprotic solvent, a member of greenhouse gas, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. Intramolecular hydroamidation of alkynes can proceed through alkyne activation by indium(III) and then 6-exo-dig cyclization, leading to a fused pyran ring with high selectivity, high atom economy, and good to excellent yields. The cyclization was accomplished through the oxygen, not the nitrogen, of the amide functional group.
The Chemistry of Water
Structure Means Function
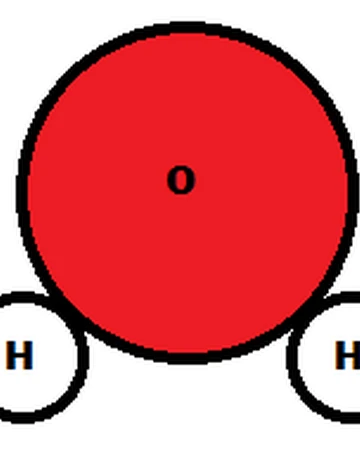
Water is a Chemical!? Indeed! Water is one of our most plentiful chemicals. Its chemical formula, H20, is probably the most well known of all chemical formulas. What does the chemical formula tell us?The formula H20 tells us that one molecule of water is comprised of 2 atoms of hydrogen and one atom of oxygen bonded together. The bonds which hold the hydrogen and oxygen together are called covalent bonds - they are very strong. Let's look at a picture of a molecule of water: In this picture the two hydrogens are represented by white spheres and the oxygen by a red sphere. IMAGE SOURCE: 'Chemistry and Life', 4th Edition, John W. Hill, Dorothy M. Feigl, and Stuart J. Baum, Macmillan Publishing Company, New York, 1993 In this second picture, the hydrogens are shown as white spheres, the oygen as a red sphere. The 'sticks' holding the hydrogens to the oxygen represent covalent bonds. Why does the water molecule look bent?The water molecule maintains a bent shape (bent at 107.5 degrees actually) because of two considerations. First the tetrahedral arrangment around the oxygen and Second the presence of lone pair electrons on the oxygen. What are Lone Pair Electrons? These are the electrons that are not involved in the covalent bonds. The pairs of electrons are left alone. In our picture they are represented by the double dots. These lone pairs are very negative - containing two negative electrons each - and want to stay away from each other as much as possible. These repulsive forces act to push the hydrogens closer togetherDid you say 'Tetrahedral' - What does that mean? Tetrahedral means 'four-sided'. In chemistry we interpret this in our imaginations. Draw the central atom in an imaginary space. Next put the atoms attached to the central atom around it such that the distance between them is maximized. The arrangement you'll adopt will be the form of a regular tetrahedron. This molecular shape is shown below. It has regular bond angles of 109.5
If we do a similar arrangement of water, putting oxygen in the center, and using the two hydrogens and two lone pairs at the corners, we also come up with a tetrahedral arrangement. However, there is one important difference - the bond angles for water are not 109.5. Because of the presence of the very negative lone pair electrons, the two hydrogens are squeezed together as the two lone pairs try to get away from each other as far as possible. The resulting angle gives water a 104.5 bond angle. Because we don't 'see' the electrons, the resulting tetrahedron 'looks' BENT! IMAGE SOURCE: 'Chemistry and Life', 4th Edition, John W. Hill, Dorothy M. Feigl, and Stuart J. Baum, Macmillan Publishing Company, New York, 1993What's your Point? Like many things in the chemical world, the shape and structure of a molecule is an important determinant of its function. The importance of the bent structure of water is that it provides water with two distinct 'sides': One side of the water molecule has two negative lone pairs, while the other side presents the two hydrogens. Let's take another look: [ fig of electron density map of water ] Does this make water unusual? YES! But it's not just that the molecule is bent that makes it special. Water is also highly polar - the two sides of water have very different charge. The lone pairs are negative - Are the Hydrogens positive? The hydrogens are slightly positive. They get this way because of the 'electronegativity' of oxygen. Electronegativity is a measure of how much one atom wants to have electrons, and oxygen wants to have electrons more than hydrogen does. Oxygen has a higher electronegativity. Because of this difference in electronegativity, the electrons in the covalent bonds between oxygen and hydrogen get pulled slightly toward the oxygen. This leaves the hydrogens a little bit electron-deficient and thus slightly positive. We can draw this polarization like this:IMAGE SOURCE: 'Chemistry in Context' Wm C Brown Publishers, Dubuque Iowa, 2nd edition, A project of the American Chemical Society, ed: A. Truman Schwartz et al., 1997, Chapter 5 'The Wonder of Water' Or looking at it from a 'net polarization' perspective, like this: IMAGE SOURCE: 'Chemistry and Life', 4th Edition, John W. Hill, Dorothy M. Feigl, and Stuart J. Baum, Macmillan Publishing Company, New York, 1993 What does the polarization have to do with the properties of water? Everything! Because water has a slightly negative end and a slightly positive end, it can interact with itself and form a highly organized 'inter-molecular' network. The positive hydrogen end of one molecule can interact favorably with the negative lone pair of another water molecule. This interaction is call 'Hydrogen Bonding'. It is a type of weak electrostatic attraction (positive to negative). Because each and every one of the water molecules can form four Hydrogen Bonds, an elaborate network of molecules is formed.IMAGE SOURCE: 'Chemistry in Context' Wm C Brown Publishers, Dubuque Iowa, 2nd edition, A project of the American Chemical Society, ed: A. Truman Schwartz et al., 1997, Chapter 5 'The Wonder of Water'But if the Hydrogen Bonds are weak, how can they be important? Think of how many there are! There is strength in numbers! The polarity also allows water interact with an electric field: And to interact with other polar molecules - which is how substances become dissolved in water. IMAGE SOURCE: 'Chemistry in Context' Wm C Brown Publishers, Dubuque Iowa, 2nd edition, A project of the American Chemical Society, ed: A. Truman Schwartz et al., 1997, Chapter 5 'The Wonder of Water' | Selected by the SciLinks program, a service of National Science Teachers Association. Copyright 1999 - 2002
|
|---|
20 - The Mystery, Art, and Science of Water
Chris Witcombe and Sang Hwang
Sweet Briar College
WATER NEWS
watermatters has moved!
May 22nd, 2020
watermatters is now located at 3622 W. 4th Ave in Vancouver, BC.
Hydrogen: What's the difference between H, H2, H+, H- and OH- ?
Distinguishing between these different forms of Hydrogen can be confusing to those of us who flunked high school chemistry. Here is an attempt at clarification.
H = Atomic Hydrogen
Atomic hydrogen is number 1 on the Periodic Table of Elements. It consists of one proton and one unpaired electron which means that it is a free radical.
However an atom of hydrogen rarely exists on its own because its unpaired electron eagerly seeks to join up with another electron.
The molecular form of hydrogen is more common.
H2 = Molecular Hydrogen
H2 is a gas which forms when two hydrogen atoms bond together and become a hydrogen molecule. H2 is also called molecular hydrogen.It consists of two protons and two electrons. Consequently it is the most common form of Hydrogen because it is stable with a neutral charge. H2 is not a free radical. It is the antioxidant in ‘hydrogen-rich' water.
H2 is the smallest molecule in the universe. That means it can go where nothing else can …including into your mitochondria which are the powerhouses of your cells. Hydrogen gas cannot be kept in plastic because it will pass right through the walls of the container.
H+ = Proton
When the Hydrogen atom loses an electron all that is left is a proton. It becomes the positively charged hydrogen ion known as H+. This is the form of Hydrogen that produces the ATP enzyme that powers our cells and mitochondria.
H2o Atomic Mass
The H+ hydrogen ion is the basis of the pH scale.
H:– = Hydride
Hydride is a hydrogen atom which has an extra electron. This means that it is a negatively charged ion, or anion. That is why Hydride ion (H-) has the minus sign distinguishing it from a regular Hydrogen atom (H). The two dots after the H means that this ion has two electrons instead of just one. The extra electron means that H- is not a free radical however it is not stable because this form of hydrogen is a very strong base (extremely alkaline) which reacts with water to produce hydroxide (OH–and molecular hydrogen (H2).
+in+the+outer+shell+of+an+atom%E2%80%99s+electron+cloud%2C+which+can+combine+with+other+atoms+to+form+molecules+*The+number+of+valence+electrons+in+an+atom+of+an+element+determines+many+properties+of+that+element%2C+including+the+ways+in+which+the+atom+can+bond+with+other+atoms..jpg)
H:– + H2O –> H2O + OH–
Hydride (H:– ) also reacts with metals to form chemical compounds which are reducing agents.
OH– = Hydroxide ion

Hydroxide (OH–) is also known as the hydroxyl ion. When water dissociates or comes apart into its component parts it forms OH– (hydroxide ions) and H3O+ (hydronium ions).
2H2O ⇆ OH– and H3O+
This reaction is reversible. The hydroxide ion also reacts with the hydronium ion (H3O+) to become two water molecules.
The Hydroxide ion (OH– ) is a base (alkaline). The Hydroxide ion is not a free radical or an antioxidant. Dissolved molecular hydrogen gas (H2) is the antioxidant in ‘hydrogen-rich' water.
Hydroxide (OH–) is sometimes confused with the hydroxyl radical (OH•). The dot to the upper right of the OH indicates an unpaired electron which means that Hydroxyl is a free radical, actually one of the most reactive oxygen radicals. Hydroxide and Hydroxl are two entirely different species. It is important to not confuse them.
H3O+ = Hydronium ion
A water molecule (H20) plus a hydrogen ion (H+) becomes a hydronium ion (H3O+). The H+ ion is a lone proton with a powerful charge. It does not exist on its own in an aqueous solution because it is immediately attracted to the unshared electrons in the oxygen atom of H2O. The result is Hydronium (H3O+). This process is reversible. Two water molecules can disassociate to form hydronium plus hydroxide.
2H2O ⇆ OH– and H3O+
Experiments indicate that the proton (H+) is very promiscuous. It changes from one H2O partner to another many times per second creating a new H3O+ ion as it moves.

The Chemistry of Water
Structure Means Function
Water is a Chemical!? Indeed! Water is one of our most plentiful chemicals. Its chemical formula, H20, is probably the most well known of all chemical formulas. What does the chemical formula tell us?The formula H20 tells us that one molecule of water is comprised of 2 atoms of hydrogen and one atom of oxygen bonded together. The bonds which hold the hydrogen and oxygen together are called covalent bonds - they are very strong. Let's look at a picture of a molecule of water: In this picture the two hydrogens are represented by white spheres and the oxygen by a red sphere. IMAGE SOURCE: 'Chemistry and Life', 4th Edition, John W. Hill, Dorothy M. Feigl, and Stuart J. Baum, Macmillan Publishing Company, New York, 1993 In this second picture, the hydrogens are shown as white spheres, the oygen as a red sphere. The 'sticks' holding the hydrogens to the oxygen represent covalent bonds. Why does the water molecule look bent?The water molecule maintains a bent shape (bent at 107.5 degrees actually) because of two considerations. First the tetrahedral arrangment around the oxygen and Second the presence of lone pair electrons on the oxygen. What are Lone Pair Electrons? These are the electrons that are not involved in the covalent bonds. The pairs of electrons are left alone. In our picture they are represented by the double dots. These lone pairs are very negative - containing two negative electrons each - and want to stay away from each other as much as possible. These repulsive forces act to push the hydrogens closer togetherDid you say 'Tetrahedral' - What does that mean? Tetrahedral means 'four-sided'. In chemistry we interpret this in our imaginations. Draw the central atom in an imaginary space. Next put the atoms attached to the central atom around it such that the distance between them is maximized. The arrangement you'll adopt will be the form of a regular tetrahedron. This molecular shape is shown below. It has regular bond angles of 109.5
If we do a similar arrangement of water, putting oxygen in the center, and using the two hydrogens and two lone pairs at the corners, we also come up with a tetrahedral arrangement. However, there is one important difference - the bond angles for water are not 109.5. Because of the presence of the very negative lone pair electrons, the two hydrogens are squeezed together as the two lone pairs try to get away from each other as far as possible. The resulting angle gives water a 104.5 bond angle. Because we don't 'see' the electrons, the resulting tetrahedron 'looks' BENT! IMAGE SOURCE: 'Chemistry and Life', 4th Edition, John W. Hill, Dorothy M. Feigl, and Stuart J. Baum, Macmillan Publishing Company, New York, 1993What's your Point? Like many things in the chemical world, the shape and structure of a molecule is an important determinant of its function. The importance of the bent structure of water is that it provides water with two distinct 'sides': One side of the water molecule has two negative lone pairs, while the other side presents the two hydrogens. Let's take another look: [ fig of electron density map of water ] Does this make water unusual? YES! But it's not just that the molecule is bent that makes it special. Water is also highly polar - the two sides of water have very different charge. The lone pairs are negative - Are the Hydrogens positive? The hydrogens are slightly positive. They get this way because of the 'electronegativity' of oxygen. Electronegativity is a measure of how much one atom wants to have electrons, and oxygen wants to have electrons more than hydrogen does. Oxygen has a higher electronegativity. Because of this difference in electronegativity, the electrons in the covalent bonds between oxygen and hydrogen get pulled slightly toward the oxygen. This leaves the hydrogens a little bit electron-deficient and thus slightly positive. We can draw this polarization like this:IMAGE SOURCE: 'Chemistry in Context' Wm C Brown Publishers, Dubuque Iowa, 2nd edition, A project of the American Chemical Society, ed: A. Truman Schwartz et al., 1997, Chapter 5 'The Wonder of Water' Or looking at it from a 'net polarization' perspective, like this: IMAGE SOURCE: 'Chemistry and Life', 4th Edition, John W. Hill, Dorothy M. Feigl, and Stuart J. Baum, Macmillan Publishing Company, New York, 1993 What does the polarization have to do with the properties of water? Everything! Because water has a slightly negative end and a slightly positive end, it can interact with itself and form a highly organized 'inter-molecular' network. The positive hydrogen end of one molecule can interact favorably with the negative lone pair of another water molecule. This interaction is call 'Hydrogen Bonding'. It is a type of weak electrostatic attraction (positive to negative). Because each and every one of the water molecules can form four Hydrogen Bonds, an elaborate network of molecules is formed.IMAGE SOURCE: 'Chemistry in Context' Wm C Brown Publishers, Dubuque Iowa, 2nd edition, A project of the American Chemical Society, ed: A. Truman Schwartz et al., 1997, Chapter 5 'The Wonder of Water'But if the Hydrogen Bonds are weak, how can they be important? Think of how many there are! There is strength in numbers! The polarity also allows water interact with an electric field: And to interact with other polar molecules - which is how substances become dissolved in water. IMAGE SOURCE: 'Chemistry in Context' Wm C Brown Publishers, Dubuque Iowa, 2nd edition, A project of the American Chemical Society, ed: A. Truman Schwartz et al., 1997, Chapter 5 'The Wonder of Water' | Selected by the SciLinks program, a service of National Science Teachers Association. Copyright 1999 - 2002
|
|---|
20 - The Mystery, Art, and Science of Water
Chris Witcombe and Sang Hwang
Sweet Briar College
WATER NEWS
watermatters has moved!
May 22nd, 2020watermatters is now located at 3622 W. 4th Ave in Vancouver, BC.
Hydrogen: What's the difference between H, H2, H+, H- and OH- ?
Distinguishing between these different forms of Hydrogen can be confusing to those of us who flunked high school chemistry. Here is an attempt at clarification.
H = Atomic Hydrogen
Atomic hydrogen is number 1 on the Periodic Table of Elements. It consists of one proton and one unpaired electron which means that it is a free radical.
However an atom of hydrogen rarely exists on its own because its unpaired electron eagerly seeks to join up with another electron.
The molecular form of hydrogen is more common.
H2 = Molecular Hydrogen
H2 is a gas which forms when two hydrogen atoms bond together and become a hydrogen molecule. H2 is also called molecular hydrogen.It consists of two protons and two electrons. Consequently it is the most common form of Hydrogen because it is stable with a neutral charge. H2 is not a free radical. It is the antioxidant in ‘hydrogen-rich' water.
H2 is the smallest molecule in the universe. That means it can go where nothing else can …including into your mitochondria which are the powerhouses of your cells. Hydrogen gas cannot be kept in plastic because it will pass right through the walls of the container.
H+ = Proton
When the Hydrogen atom loses an electron all that is left is a proton. It becomes the positively charged hydrogen ion known as H+. This is the form of Hydrogen that produces the ATP enzyme that powers our cells and mitochondria.
H2o Atomic Mass
The H+ hydrogen ion is the basis of the pH scale.
H:– = Hydride
Hydride is a hydrogen atom which has an extra electron. This means that it is a negatively charged ion, or anion. That is why Hydride ion (H-) has the minus sign distinguishing it from a regular Hydrogen atom (H). The two dots after the H means that this ion has two electrons instead of just one. The extra electron means that H- is not a free radical however it is not stable because this form of hydrogen is a very strong base (extremely alkaline) which reacts with water to produce hydroxide (OH–and molecular hydrogen (H2).
H:– + H2O –> H2O + OH–
Hydride (H:– ) also reacts with metals to form chemical compounds which are reducing agents.
OH– = Hydroxide ion
Hydroxide (OH–) is also known as the hydroxyl ion. When water dissociates or comes apart into its component parts it forms OH– (hydroxide ions) and H3O+ (hydronium ions).
2H2O ⇆ OH– and H3O+
This reaction is reversible. The hydroxide ion also reacts with the hydronium ion (H3O+) to become two water molecules.
The Hydroxide ion (OH– ) is a base (alkaline). The Hydroxide ion is not a free radical or an antioxidant. Dissolved molecular hydrogen gas (H2) is the antioxidant in ‘hydrogen-rich' water.
Hydroxide (OH–) is sometimes confused with the hydroxyl radical (OH•). The dot to the upper right of the OH indicates an unpaired electron which means that Hydroxyl is a free radical, actually one of the most reactive oxygen radicals. Hydroxide and Hydroxl are two entirely different species. It is important to not confuse them.
H3O+ = Hydronium ion
A water molecule (H20) plus a hydrogen ion (H+) becomes a hydronium ion (H3O+). The H+ ion is a lone proton with a powerful charge. It does not exist on its own in an aqueous solution because it is immediately attracted to the unshared electrons in the oxygen atom of H2O. The result is Hydronium (H3O+). This process is reversible. Two water molecules can disassociate to form hydronium plus hydroxide.
2H2O ⇆ OH– and H3O+
Experiments indicate that the proton (H+) is very promiscuous. It changes from one H2O partner to another many times per second creating a new H3O+ ion as it moves.
pH = Potential of Hydrogen
pH stands for potential of Hydrogen and is actually a measurement of the concentration of hydrogen ions (H+) in a solution. Water breaks down (dissociates) into protons (H+) and hydroxides (OH–). This reaction is reversible.
H2o Atom Model
H2O ⇆ H+ and OH–
2H2O ⇆ OH– and H3O+
H2o Atomic Mass
pH indicates whether water is acidic, neutral, or alkaline. More H+ = more acidic. Less H+ = more alkaline.
Because H+ immediately associates with H2O to form H3O+ (Hydronium), pH can also be said to be a measurement of the concentration of H3O+ in a solution.
H2o Atom Model
The pH scale is logarithmic. Increasing by 1 on the pH scale results in a 10 times decrease in the hydronium ion concentration and increasing by 3 on the pH scale results in a 1,000 times decrease in the hydronium ion concentration.
- adidas store Says:
adidas store
I've been surfing on-line more than three hours lately, but I by no means discovered any interesting article like yours. Itˇs pretty price enough for me. Personally, if all website owners and bloggers made excellent content material as you probably di…
- air max nike mujer Says:
air max nike mujer
Well I sincerely enjoyed reading it. This post provided by you is very useful for correct planning.
- Sneakers Isabel Marant Baratas Says:
Sneakers Isabel Marant Baratas
You actually make it seem so easy with your presentation but I find this matter to be really something that I think I would never understand. It seems too complex and extremely broad for me. I am looking forward for your next post, I抣l try to get the h…

